Clinical Trial Management System Market - By Component (Software, Services), By Product (Enterprise-based, Site-based), By Delivery Mode (Web, Cloud, On Premise), By End-use (Pharma & Biopharma Companies, CROs) & Forecast, 2022-2030
Published Date: August - 2024 | Publisher: MIR | No of Pages: 240 | Industry: Healthcare | Format: Report available in PDF / Excel Format
View Details Buy Now 2890 Download Sample Ask for Discount Request CustomizationClinical Trial Management System Market Size
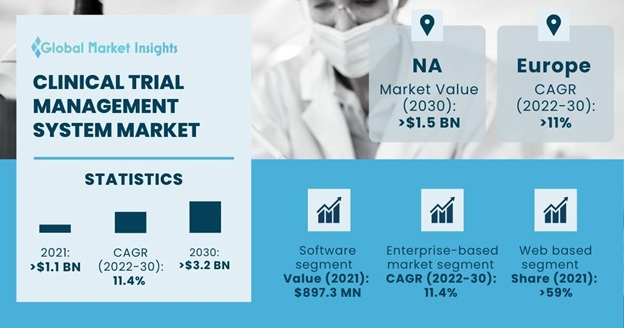
Clinical Trial Management System Market size accounted for about USD 1.2 billion in 2021 and is estimated to grow with a 11.4% market CAGR between 2022 to 2030, owing to the rising focus of the healthcare sector in research and development activities worldwide.
Many large-scale pharmaceutical and biotech industry participants are noted to be engaged in constant research in order to develop novel products as well as to sustain in the market. For instance, according to the UNESCO Institute for Statistics (UIS), in 2021, the global R&D investment surpassed USD 1.7 trillion. Around ten countries accounted for nearly 80% of all R&D spending.
| Report Attribute | Details |
|---|---|
| Base Year | 2021 |
| Clinical Trial Management System Market Size in 2021 | USD 1,169.9 Million |
| Forecast Period | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR | 11.4% |
| 2030 Value Projection | USD 3,202.7 Million |
| Historical Data for | 2017 to 2021 |
| No. of Pages | 150 |
| Tables, Charts & Figures | 225 |
| Segments covered | Component, Product, Delivery Mode, End-use and Region |
| Growth Drivers |
|
| Pitfalls & Challenges |
|
Several countries have committed to further enhance the public and corporate R&D spending, as well as the number of researchers, by 2030 as part of the Sustainable Development Goals (SDGs). Furthermore, one of the primary value drivers for research-based pharmaceutical businesses is innovative medications addressing the unmet medical needs. Owing to enormous growth in R&D activities, having essential tools to plan, manage and track clinical studies effectively is need of the hour. Hence, eClinical solutions, such as clinical trial management system (CTMS), are gaining market prominence.
The emergence of the COVID-19 pandemic significantly impacted the overall healthcare sector, disrupting the activities of virtually every organization operating within. To conduct clinical trials smoothly in healthcare and life science industry, where physical access to patients was not possible, using new ways for patient monitoring and caring had become necessary. Due to which the previously present data collection methods, CTMS which were not utilized before, were quickly adopted in clinical trials during COVID outbreak.
Although, these means were available before, the pandemic acted as a catalyst in their increased market usage. With the introduction of CTMS, new approaches and technology in clinical trials specifically the one’s related to remote data collection from patients increased the data variety and volume. As a result, majority positive impacts of clinical trial approaches newly implemented during the pandemic were due to the data. The respondents using CTMS and other new methods of data collection in clinical trials found their data to be well timed, accurate, frequent, and more robust. However, many clinical trials were suspended or postponed due to imposed lockdowns and other restrains due to government rules.
Increasing clinical trial activity is expected to spur the market statistics
The number of clinical trials has significantly increased in the past two decades. This is because of increasing penetration of cutting-edge medical technologies coupled with the rising demand for innovative medications with higher efficacy.
For instance, as of June 2022, according to the U.S. National Library of Medicine, 419,632 clinical studies were registered globally, with nearly 77% interventional studies and 22% observational studies. Also, according to the WHO International Clinical Trials Registry Platform (ICTRP), during the period 1999-2021, the U.S. (157,618) had the maximum clinical trials registered, followed by China (80,333), and Japan (57,754).
Clinical trial management system is both cost and time effective, as these are employed to collect and organise data and can be shared with several care providers and dispersed across multiple systems. These technologies can help with site identification and recruiting, as well as control and tracking of participant enrolment and the database of participants. Therefore, increasing clinical trial volume is contributing to the rising market demand for such CTMS solutions to effectively manage the clinical studies.
High cost of clinical trial management system may hamper the market demand
The high costs associated with clinical trial management system can potentially limit its market growth. Procurement, installation, and maintenance cumulatively surge the overall cost required for the adoption of CTMS solutions. On premise and SaaS-based systems both include installation fees. Additional setup, customization costs are charged to companies based on the distinctive organizational and study-specific demands. There are different cost structures observed with respect to number of users and number of sites under consideration. Certain contract or commitment term fees also need to be considered for CTMS along with periodic maintenance and upgradation of systems.
Clinical Trial Management System Market Analysis
Based on component, the market for clinical trial management system is classified into software and services. The software segment accounted for USD 897.3 million revenue in 2021 and is expected to dominate from 2022 to 2030 due to its high utilization and increased adoption in clinical trials. Various biotechnology, pharmaceutical companies and other life science organizations promoting research constantly conduct complex clinical trials. These trials are aimed at discovering novel medicines and medical devices.
With the increasing clinical trials activity and multi-site clinical trials gaining momentum, a need for effective tools to manage the studies with ease, maintain data with accuracy and manage the operational aspects of clinical studies also increases. Using software-based tools enables the sponsors to manage clinical trials efficiently.

The market for clinical trial management system is segmented by product into enterprise-based and site-based. The enterprise-based market segment is expected to witness a lucrative CAGR of 11.4% between 2022 and 2030. Enterprise based clinical trial management system is versatile and can be customized as per requirements. Such clinical trial management system feature functionalities related to documents, worksheets, calendars, schedules, contacts, payments, milestones etc. Enterprise based clinical trial management system solutions are also noted to be scalable to cater to the requirements of small, medium, and large-scale organizations. Therefore, the enterprise-based clinical trial management system market is expected to witness a significant demand throughout the forecast period.
Based on delivery mode, the clinical trial management system market value is segmented into web based, cloud based and on-premises. Web based segment dominated the global market accounting for over 59% market proportion in 2021 and is expected to witness lucrative growth over the forecast period. The web-based is highly effective, due to the ability of being adopted for diverse study designs and having multiple functionalities.
The clinical trial management system market share is segmented on the basis of end-use into pharmaceutical and biopharmaceutical companies and clinical research organization, among others. The pharmaceutical and biopharmaceutical companies are expected to register a healthy growth rate of 10.6% over the forecast period. The increasing demand for developing novel products for chronic illnesses as well as innovative medical devices compels the manufacturers/sponsors to significantly invest in research & development activities, including drug discovery and clinical trials.
Further, with an increasing number of novel diseases, such as coronavirus, spreading across geographies further propels the demand for developing targeted diagnostic devices as well as vaccines and related medications. Additionally, the increasing competition among large-scale pharma companies also emphasizes on novel product development to enhance the product portfolios.

North America clinical trial management system market is anticipated to surpass USD 1.5 billion by 2030. The rising clinical trial activity in North America coupled with higher cost of clinical operations in the region as compared to countries of Asia Pacific and Latin America are some of the prime factors for the regional dominance in the global CTMS market.
For instance, according to the WHO International Clinical Trials Registry Platform (ICTRP), between 1999 and 2021, the U.S. reported the highest number of clinical trials. It is evident that major pharma and biotech companies in the region heavily invest in research activities and are competing to discover and develop innovative medicines with greater efficacy. Also, well defined government regulations and support to the pharmaceutical industry is expected to encourage the clinical trials. These factors, coupled with a significantly high adoption rate for integrating digital solutions throughout clinical trials leads to an increasing employment of CTMS. CTMS also enables to organize & evaluate the clinical study data for regulatory submissions.
Clinical Trial Management System Market Share
Major players operating in the clinical trial management system industry include
- Clario
- Advarra
- Datatrak Inc
- PHARMASEAL
- SimpleTrials
- MasterControl Inc
- Medidata Solution Inc (Dassault Systemes)
- MedNet Solution Inc
- Merge Healthcare (IBM Watson)
- Anju Software Inc
- Oracle Corporation
- Paraxel International
- Veeva System
These players opt for various inorganic growth strategies such as partnerships, collaborations, mergers and acquisitions and product launch to create a global footprint and industry competition.
Some of the recent industry developments
- In April 2021, ERT, a global leader in clinical endpoint data solutions, merged with Bioclinica and name of this combined company is changed to Clario. This merger combined Bioclinica's expertise of imaging, eClinical software and drug safety solutions with ERT's expertise of eCOA, cardiac safety, respiratory and wearables. The development was targeted at combining the technological aspects of both the companies to offer an enhanced service to a wider customer base.
- In March 2021, Advarra, a company offering solutions to make clinical trials safer smarter and faster announced the completion of its acquisition of Bio-Optronics, creator of CCTrialSuite and which offers clinical trial management system (CTMS) solutions for healthcare system and clinical research. This acquisition strategy enabled the company to enhance its product portfolio and increase its customer base.
The clinical trial management system market report includes an in-depth coverage of the industry with estimates & forecast in terms of revenue in USD from 2022 to 2030, for the following segments
Click here to Buy Section of this Report
By Components
- Software
- Services
By Product
- Enterprise-based
- Site-based
By Delivery Mode
- Web CTMS
- Cloud CTMS
- On Premise CTMS
By End-use
- Pharmaceutical & Biopharmaceutical Companies
- Clinical Research Organizations (CROs)
- Others
The above information is provided for the following regions and countries
By Region
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Asia Pacific
- China
- India
- Japan
- Australia
- Latin America
- Brazil
- Mexico
- Middle East & Africa
- South Africa
- Saudi Arabia
Related Reports
- Pet Fitness Care Market - By Component (Services [Physio Assessments, Agility & Gym Sessions, Aquatic Sessions, Outdoor ...
- Veterinary Infusion Pumps Market – By Product (Syringe, Volumetric, Ambulatory, Implantable), Animal type (Companion, ...
- Veterinary CRO and CDMO Market – By Animal Type (Companion, Livestock), Service Type (CRO, [Clinical Trials, Quality A...
- Pet Sitting Services Market – By Service (In-home Pet Sitting Services, Drop-in Visits, Boarding Services, Daycare Ser...
- Veterinary Video Endoscopes Market – By Product (Equipment, PACS Software), Animal Type (Small, Large), Application (D...
- Pet Herbal Supplements Market – By Product Type (Multivitamins & Minerals, Omega 3 Fatty Acids), Application (Digestiv...
Table of Content
To get a detailed Table of content/ Table of Figures/ Methodology Please contact our sales person at ( chris@marketinsightsresearch.com )
List Tables Figures
To get a detailed Table of content/ Table of Figures/ Methodology Please contact our sales person at ( chris@marketinsightsresearch.com )
FAQ'S
For a single, multi and corporate client license, the report will be available in PDF format. Sample report would be given you in excel format. For more questions please contact:
Within 24 to 48 hrs.
You can contact Sales team (sales@marketinsightsresearch.com) and they will direct you on email
You can order a report by selecting payment methods, which is bank wire or online payment through any Debit/Credit card, Razor pay or PayPal.
Discounts are available.
Hard Copy