Point of Care Molecular Diagnostics Market Size
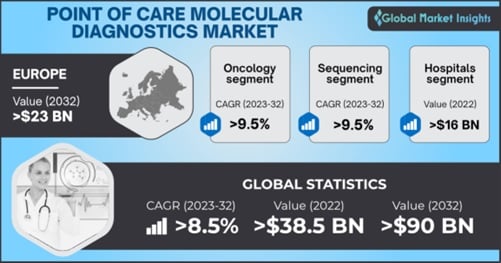
Point of Care Molecular Diagnostics Market size was valued at more than USD 38.5 billion in 2022 and is projected to grow substantially at over 8.5% growth rate from 2023 to 2032 owing to the increasing efforts for advancing diagnostic procedures.
R&D initiatives for improving diagnostic procedures for easy analysis of complex and severe diseases are foreseen to stimulate industry expansion. Moreover, the increasing number of favorable government programs supporting the development of molecular diagnostics owing to their medical, economic, and societal advantages are projected to augment point of care molecular diagnostics market revenues.
Point of Care Molecular Diagnostics Market Report Attributes
| Report Attribute |
Details |
| Base Year | 2022 |
|---|
| Point of Care Molecular Diagnostics Market Size in 2022 | USD 38.5 Billion |
|---|
| Forecast Period | 2023 to 2032 |
|---|
| Forecast Period 2023 to 2032 CAGR | 8.5% |
|---|
| 2032 Value Projection | USD 90 Billion |
|---|
| Historical Data for | 2018 to 2022 |
|---|
| No. of Pages | 170 |
|---|
| Tables, Charts & Figures | 174 |
| Segments covered | Technology, Application, End-use, and Region |
|---|
| Growth Drivers | - Increasing incidence of infectious diseases
- Increasing R&D funding to reduce time required for molecular diagnostic tests
- Growing awareness about pre-disease diagnostics to control occurrence of diseases
- Technological advancements in DNA sequencing and analysis
|
|---|
| Pitfalls & Challenges | - Long product development time
- Complex regulatory process
|
|---|
Complicated regulatory processes may slow down market progress
The business growth is hampered by complicated regulatory procedures such as pre- and post-market clearances and multiple clinical testing. For instance, the Clinical Laboratory Improvement Amendments (CLIA) rules mandate that the QC (Quality Control) for illness testing must be completed in accordance with the manufacturer’s instructions. In the absence of a specification from the manufacturer, the testing facility must establish a policy that adheres to acceptable laboratory procedures. However, with the growing awareness about the product’s benefits among buyers, the government may relax the requirements for adhering to strict laws, which is foreseen to benefit the market outlook.
Point of Care Molecular Diagnostics Market Analysis
By technology, the point of care molecular diagnostics market share from the sequencing segment is anticipated to record growth at more than 9.5% CAGR over 2023-2032. The development of higher-quality genetic sequencing is favorably influencing segment development. Additionally, favorable government policies promoting the adoption of modern technology are expected to bolster segment progress.
In terms of end-use, the point of care molecular diagnostics market is segmented into hospitals, clinics, diagnostic centers, and others. The hospitals segment accounted for a valuation of over USD 16 billion in 2022, the report claims. Easy access to healthcare professionals and the surging availability of advanced medical equipment and treatments are the major factors estimated to propel industry outlook. In November 2021, healthcare giant Roche launched cobas® 5800 System, a molecular laboratory device that assists hospitals and laboratories in addressing challenges brought on by increasing patient testing and reimbursement complexities.
By application, the point of care molecular diagnostics market from oncology segment is projected to grow at over 9.5% CAGR through 2032 attributed to the high prevalence of cancer cases and rising healthcare expenditure. According to the National Center for Biotechnology, China was estimated to have 4,820,000 new cancer cases in 2022. Increasing regulatory approvals for oncology PoC molecular diagnostic products further is set to contribute to segment growth. In August 2022, Canhelp Genomics, a Chinese genetic diagnostics business, attained National Medical Products Administration (NMPA) approval for its Canhelp-Origin pan-cancer test and companion analytic software.

Europe point of care molecular diagnostics market valuation is estimated to reach over USD 23 billion by 2032 owing to the proliferation of molecular technology for the rising need for affordable, quick, and portable molecular diagnostics. Additionally, the emergence of several significant market participants is expected to fuel the regional market revenue. For instance, in March 2022, Sense Biodetection (Sense), a pioneer in molecular diagnostics pioneer received the CE Marking in Europe, for its Veros COVID-19, a fully integrated, easy-to-use molecular diagnostic test that offers laboratory-quality results in 15 minutes.
Point of Care Molecular Diagnostics Market Share
Some leading companies operating in the point of care molecular diagnostics market are
- BioMerieux SA
- Danaher Corporation
- Bio-Rad Laboratories
- Sysmex Corporation
- Roche Diagnostics
- OraSure Technologies, Inc
- VIRCELL S.L.
Several new advancements and product range expansion efforts by industry players are estimated to be observed in the market. In July 2022, OraSure Technologies, Inc., a global leader in point of care diagnostic testing technologies, announced the availability of the OMNIgene•GUT DNA and RNA product (OMR-205) to gut microbiome researchers, allowing them insight into gut microbes gene expression.
Impact of COVID-19 Pandemic
The COVID-19 outbreak positively influenced the point of care molecular diagnostics industry expansion. The demand for molecular diagnostic goods increased significantly between March 2020 and October 2020 and is anticipated to continue increasing as a result of the conditions surrounding uncontained diseases. In addition, the mounting requirement for parts used in molecular diagnostics prompted manufacturers to boost their production capacity, thereby positively influencing the market outlook. For example, BGI, a key player in in-vitro diagnostic devices, exported millions of test kits and supplied operating models and equipment worldwide
The point of care molecular diagnostics market research report includes in-depth coverage of the industry with estimates & forecast in terms of revenue in USD Million from 2018 to 2032 for the following segments
Click here to Buy Section of this Report
By Technology
- PCR
- In situ hybridization
- Sequencing
- Isothermal amplification
- Others
By Application
- Infectious Disease
- Flu
- Respiratory Syncytial Virus (RSV)
- Tuberculosis (TB)
- HIV
- Gonorrhoea
- Chlamydia
- Hepatitis C
- Hepatitis B
- COVID-19
- Others
- Oncology
- Hematology
- Others
By End-use
- Hospitals
- Clinics
- Diagnostic centers
- Others
The above information is provided for the following regions and countries
- North America
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Russia
- Others
- Asia Pacific
- China
- India
- Japan
- South Korea
- Malaysia
- Thailand
- Vietnam
- Philippines
- Australia
- Others
- Latin America
- Brazil
- Mexico
- Argentina
- Others
- Middle East and Africa
- South Africa
- Egypt
- Nigeria
- Morocco
- Saudi Arabia
- UAE
- Others



