Transradial Access Devices Market Size
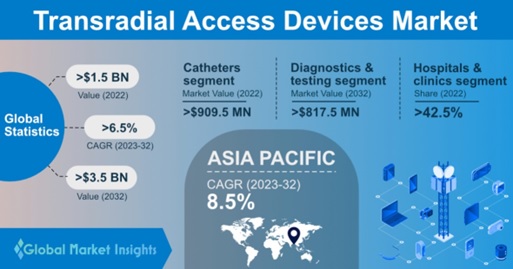
Transradial Access Devices Market size was registered at more than USD 1.5 billion in 2022. The industry is slated to grow at over 6.5% CAGR from 2023 to 2032 as a result of the widespread adoption of unhealthy habits, such as alcohol and smoking, along with sedentary lifestyles.
A significant rise in incidences of cardiovascular disease in developed and developing economies is expected to fuel market progress. Moreover, as per the Journal of the American College of Cardiology study, projected rates of cardiovascular risk factors and disease are set to increase substantially in the U.S. by 2060.
Furthermore, the mounting geriatric population base across the globe is also bolstering the demand for TRA devices, thereby augmenting market revenues. Heart-related conditions such as congestive heart failure, coronary artery disease, hypertension, atrial fibrillation, etc. are more common in older adults. According to the Population Reference Bureau, the number of persons aged 65 and above is expected to rise from 52 million in 2018 to almost 95 million by 2060.
Transradial Access Devices Market Report Attributes
| Report Attribute |
Details |
| Base Year | 2022 |
|---|
| Transradial Access Devices Market Size in 2022 | USD 1.5 Billion |
|---|
| Forecast Period | 2023 to 2032 |
|---|
| Forecast Period 2023 to 2032 CAGR | 6.5% |
|---|
| 2032 Value Projection | USD 3.5 Billion |
|---|
| Historical Data for | 2018 to 2022 |
|---|
| No. of Pages | 120 |
|---|
| Tables, Charts & Figures | 177 |
| Segments covered | Product, Application, End-use, and Region |
|---|
| Growth Drivers | - Increasing prevalence of cardiovascular disease in developed and developing economies
- Growing geriatric population base globally
- Rising preference for interventional procedures using radial artery access
- Growing use of radial access devices in pediatric patients
|
|---|
| Pitfalls & Challenges | - Stringent regulatory framework
- High cost and maintenance of vascular access devices
- Dearth of cardiothoracic surgeons in developing nations
|
|---|
Stringent regulations may restrain industry progression
A strict regulatory framework is a major factor hampering transradial access devices market revenue. In the U.S., guidelines for peripheral, coronary, and neurovascular devices, including guidewires and catheters, are created by the Centre for Devices and Radiological Health (CDRH). The U.S. FDA rules for patient safety push all medical devices to meet certain criteria. Manufacturers lose time and money if approvals are postponed or rejected. However, supportive initiatives toward healthcare system modification are aiding the market outlook.
Transradial Access Devices Market Analysis
With respect to the product, the transradial access devices market is divided into catheters, sheath & sheath introducers, guidewires, and accessories. The catheters segment was valued at more than USD 909.5 million in 2022. An upsurge in vascular intervention procedures is set to drive segment size. Additionally, radial-specific catheters allow simultaneous angiography of both right and left coronary arteries by clockwise or anticlockwise rotation. A September 2021 research study states that a catheter system eases anatomies and is a cost-effective access site care compared to conventional coronary interventional procedures.
|
Global Transradial Access Devices Market Share, By Application, 2022 (%)
|
|
Segment
|
Global market share (%), 2022
|
|
Drug administration
|
36.50%
|
|
Fluid and nutrition administration
|
26.30%
|
|
Blood transfusion
|
12.60%
|
|
Diagnostics and testing
|
20%
|
|
Others
|
4.70%
|
When it comes to using transradial access devices, there are a few different ways they're commonly usedgiving fluids and nutrients, doing tests and diagnostics, transfusing blood, administering medications, and some other things. Out of all these uses, the one that involves testing and diagnostics is expected to really take off in the coming years, with experts predicting it could bring in over 817.5 million dollars by 2032. One big reason for this growth is that more and more adults and kids are getting heart problems, which means there's a growing need for better ways to find and treat these issues. For example, transradial access devices are being used more and more for cardiac catheterization, a procedure that helps doctors see what's going on inside the heart and treat problems like blocked arteries. In the United States alone, around 1,500 people under the age of 25 die every year from sudden heart problems. So, it's clear that there's a real need for better ways to detect and treat heart conditions, and transradial access devices are playing a big role in meeting that need.
Based on end-use, the TRA devices market is categorized into hospitals & clinics, ambulatory surgical centers, and others. The hospitals & clinics segment accounted for over 42.5% business share in 2022. The segment revenue share is likely to be stimulated by the increasing use of minimally invasive procedures, the availability of cutting-edge medical facilities, and favorable reimbursement regulations for surgeries carried out in hospital settings. For instance, in October 2022, Emblemhealth, a health insurance company, introduced a new reimbursement policy for billing in hospital facility treatment rooms.

The transradial access devices market in the Asia Pacific is primed to expand at an 8.5% CAGR over 2023-2032. This is attributed to the growing geriatric population in countries such as India and China, making it a hotspot for cardiovascular diseases, thereby increasing the demand for coronary interventions in the region and fueling business expansion. According to United Nations ESCAP, there are over 630 million people aged above 60 years in the APAC region, constituting over 60% of the global elderly population.
Transradial Access Devices Market Share
- Terumo Corporation
- Medtronic
- Boston Scientific Corporation
- Becton
- Dickinson and Company
- AngioDynamics
- Merit Medical System, Inc.
- Edwards Lifesciences Corporation
- Ameco Medical Industries
- NIPRO Medical Corporation
- Smiths Group
- OSCOR Inc
- Teleflex Corporation
are some key companies functioning in the transradial access devices industry. These firms majorly engage in extensive R&D programs and acquisitions to stay ahead in the competitive landscape.
Impact of the COVID-19 pandemic
The COVID-19 pandemic shook up the market for medical devices called TRA devices. Strict lockdowns meant people couldn't go to hospitals for non-urgent care, and the rise in COVID cases led to many surgeries being postponed. This really hurt the TRA device industry. But now, things are starting to look up again. Surgeries are being rescheduled, and the number of heart issues has actually gone up in some people who have had COVID-19. A study in the UK even found that folks who had COVID had a higher chance of getting heart problems right after getting over the virus. With the worst of the pandemic behind us, the TRA device industry is starting to get back on track. As more and more people get vaccinated and the number of COVID cases goes down, we can expect to see the industry pick up the pace and reach its pre-pandemic levels.
The transradial access devices market research report includes an in-depth coverage of the industry with estimates & forecast in terms of revenue in USD from 2018 to 2032, for the following segments
Click here to Buy Section of this Report
By Product
- Catheters
- Guidewires
- Sheath and sheath introducers
- Accessories
By Application
- Drug administration
- Fluid and nutrition administration
- Blood transfusion
- Diagnostics and testing
- Others
By End-use
- Hospitals & clinics
- Ambulatory surgical centers
- Others
The above information is provided for the following regions and countries
North America
Europe
- Germany
- UK
- France
- Italy
- Spain
Asia Pacific
- China
- India
- Japan
- Australia
Latin America
Middle East & Africa
- South Africa
- Saudi Arabia



