Contract Research Organization Market Size
Contract Research Organization Market size accounted for USD 56.7 billion in 2022 and is estimated to grow at 6.9% CAGR between 2023 and 2032. Rising adoption of advanced technologies for efficient R&D outcomes and the increasing outsourcing trends in the clinical trials industry has largely influenced the global market growth.
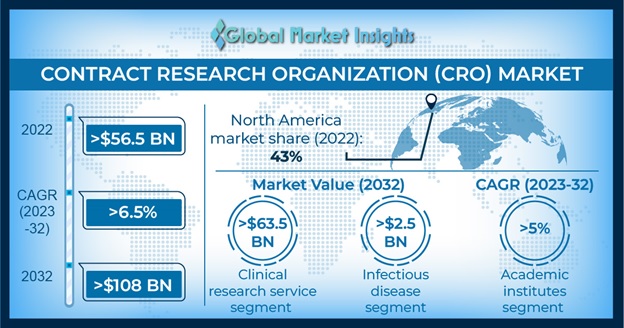
The significant shift from maintaining manual/paper-based records to digital data capture technologies is largely transforming and propelling the market growth. Moreover, the advent of COVID-19 has largely triggered the adoption of digital resources across clinical trials enhancing the quality of data while allowing greater flexibility. Furthermore, digital technologies can transform the companies’ approach towards clinical development by incorporating valuable insights from multiple sources and increasing the volume of data collected in trials enhancing clinical trial productivity, thereby fuelling the market expansion.
Contract research organization, also known as clinical research organization, is a company that assists in managing complex clinical research & trial responsibilities, clinical development & commercialization of new products for sponsor companies and drug discovery primarily in the biotechnology, medical device and pharmaceutical industries. These organizations offer services such as clinical trial management, preclinical research, clinical research, commercialization, and pharmacovigilance among other research services. These are outsourced on contract basis, also known as fee-for-service basis.
Contract Research Organization Market Report Attributes
| Report Attribute |
Details |
| Base Year | 2022 |
|---|
| Contract Research Organization Market Size in 2022 | USD 56,5 Billion |
|---|
| Forecast Period | 2023 to 2032 |
|---|
| Forecast Period 2023 to 2032 CAGR | 6.5% |
|---|
| 2032 Value Projection | USD 108 Billion |
|---|
| Historical Data for | 2018 to 2022 |
|---|
| No. of Pages | 287 |
|---|
| Tables, Charts & Figures | 406 |
| Segments covered | Service, Therapeutic area, End-use and Region |
|---|
| Growth Drivers | - Increasing R&D expenditure worldwide
- Growing number of clinical trials in emerging countries
- Growing outsourcing of R&D activities
- Rising technological advancements
|
|---|
| Pitfalls & Challenges | - Intellectual property rights issues
- Stringent regulatory policies
|
|---|
Increasing R&D expenditure worldwide will spur the CROs industry growth
The number of people with serious long-term illnesses, like heart disease and diabetes, is rising all over the world. This is largely because more and more people are living sedentary lifestyles, eating unhealthy foods, and smoking or chewing tobacco. The COVID-19 and Ebola outbreaks have also led to increased spending on research and development around the world. Drug companies are also investing heavily in research to develop new treatments for these diseases. This is because there are still many unmet medical needs. Governments and private organizations are also investing more money in research, which is helping to drive demand for contract research organizations (CROs). CROs help drug companies conduct clinical trials and other studies, which are essential for developing new drugs and treatments.
Intellectual property issues may hamper the contract research organization market statistics
Protecting intellectual property (like patents) is a big concern when it comes to outsourcing clinical trials. This is because many medical devices and drugs are patented, meaning they're protected under the law. So, if you outsource these trials to a contract research organization (CRO), there's always a risk of information leaking out. On top of that, drug companies have to share data about their products' safety and effectiveness with government agencies to get approval. This can be a lot of sensitive information that companies don't want to risk getting out. All these concerns can make it harder for CROs to grow their businesses. But on the bright side, it's also forced CROs to improve their security and operations. It's made them more efficient and helped them get rid of bad patents. It's important for CROs to make sure they have clear agreements in place with their clients. This will help protect both parties from any disputes. The government is also keeping a close eye on clinical trials, so CROs need to be transparent and follow all the rules. If they don't, it can hurt their reputation and make it harder for them to succeed.
Contract Research Organization Market Analysis
The contract research organization (CRO) market is like a big pie, and it's sliced up into different sections based on the type of services offered. One of the biggest slices is for clinical research services, which is all about testing out new drugs and treatments in people. This slice is expected to grow even bigger in the coming years, because more and more diseases are popping up, and people are looking for new ways to treat them. Within the clinical research services slice, there are even smaller slices for different phases of testing, like Phase I, II, III, and IV. These phases represent different stages in the process of developing and testing a new treatment. The whole CRO market is growing because more and more companies need help testing their drugs and treatments. These companies offer a wide range of services, from helping with early-stage development to getting regulatory approvals for new products.
Based on therapeutic area, the contract research organization market is bifurcated into oncology, clinical pharmacology, cardiology, infectious disease, neurology, gastroenterology & hepatology, ophthalmology and others. Oncology segment contributed USD 30.85 billion revenue in 2022. Increasing prevalence of cancer has compelled sponsors to focus on developing different therapies and medical devices for better management of cancer. Hence, leading to an increased number of clinical trials and discovery of various drugs for the cancer treatment.
When it comes to the companies that use contract research organizations (CROs), there are three main typesdrug makers (pharmaceutical & biopharmaceutical companies), medical device companies, and universities (academic institutes). Out of these three, drug makers are expected to lead the way with a solid growth rate of 7.4% over the next few years. Why? Because more and more drug companies are outsourcing their research and development (R&D) to CROs. The demand for CROs is also being boosted by the growing number of clinical trials and the trend towards outsourcing these trials. Plus, governments are chipping in with more money and support for clinical trials, which is expected to give the CRO industry an extra boost.

North America contract research organization market surpassed USD 24.4 billion in 2022 and is projected to register significant growth throughout the forecast period. The significant market growth is attributed to higher density of pharmaceutical and biotechnology companies across the North America region. Moreover, increasing focus of pharmaceutical and biotechnology for outsourcing clinical trials for treatment of various disease is expected to fuel the North American market size.
Contract Research Organization Market Share
Major market players operating in the contract research organization industry include
- Charles River Laboratories, Inc.
- Laboratory Corporation of America Holdings
- IQVIA
- Pharmaceutical Product Development
- PAREXEL International and Syneos Health
Mergers & acquisitions, partnerships & collaboration are some of the most adopted business strategies observed to sustain market position.
Some of the recent industry developments
- In March 2022, argenx SE and IQVIA collaborated to develop new indications for VYVGART for the treatment of myasthenia gravis in adult patients. The collaboration is anticipated to support clinical development, commercial, regulatory, and real-world evidence strategy to accelerate the development of new indications for VYVGART. This strategy enhances the company’s long-term business collaborations while expanding its customer base.
Impact of the COVID-19 pandemic
When the COVID-19 pandemic hit, it upended the world of clinical research. Lockdowns and travel restrictions put a big dent in clinical trials around the globe. But as time went on, things started to get back on track. Clinical trials started up again, and many of them focused on developing a vaccine for COVID-19. So, while the pandemic put a damper on the clinical research industry at first, it ended up boosting the market in the long run. That's because there's now a huge demand for new vaccines and drugs to fight the virus. And that's led to a lot of biotech and pharmaceutical companies signing on with contract research organizations (CROs) to help them with their research.
The contract research organization (CRO) market research report includes an in-depth coverage of the industry with estimates & forecast in terms of revenue in USD from 2018 - 2032, for the following segments
Click here to Buy Section of this Report
By Service Type
- Early phase development services
- Discovery services
- Chemistry, manufacturing & control (CMC)
- Preclinical services
- Pharmacokinetics/Pharmacodynamics (PK/PD)
- Toxicology services
- Others
- Clinical research services
- Phase I
- Phase II
- Phase III
- Phase IV
- Laboratory services
- Bioanalytical testing services
- Analytical testing services
- Physical Characterization
- Raw Material Testing
- Batch Release Testing
- Stability Testing
- Others
- Regulatory consulting services
By Therapeutic Area
- Oncology
- Clinical pharmacology
- Cardiology
- Infectious disease
- Neurology
- Gastroenterology & Hepatology
- Ophthalmology
- Others
By End-use
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Companies
- Academic Institutes
The above information is provided for the following regions and countries
- North America
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Asia Pacific
- Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia



