HPV Testing and PAP Test Market Size
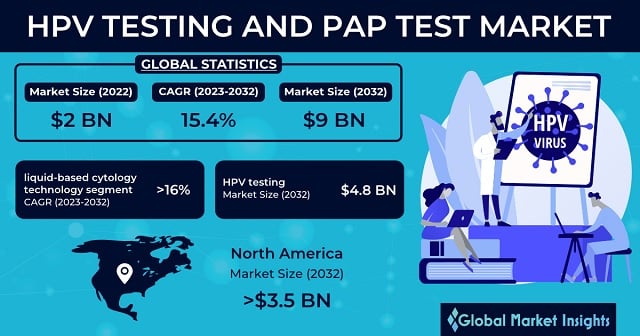
HPV Testing and PAP Test Market size was USD 2 billion in 2022 and is poised to observe over 15.4% CAGR from 2023-2032. Rising frequency of cervical cancer to drive global industry expansion.
The surging burden of cervical cancer worldwide will increase the demand for HPV testing and PAP tests. The growing popularity of screening test kits will further influence the industry expansion. To state an instance, Roche, in 2021, received the U.S. FDA approval for the Cobas HPV test deployed for detecting the risk of cervical cancer among women by identifying the presence of high-risk human papillomavirus (HPV) DNA in cervical samples.
HPV Testing and PAP Test Market Report Attributes
| Report Attribute |
Details |
| Base Year | 2022 |
|---|
| HPV Testing and PAP Test Market Size in 2022 | USD 2 Billion |
|---|
| Forecast Period | 2023 to 2032 |
|---|
| Forecast Period 2023 to 2032 CAGR | 15.4% |
|---|
| 2032 Value Projection | USD 9 Billion |
|---|
| Historical Data for | 2018 to 2022 |
|---|
| No. of Pages | 290 |
|---|
| Tables, Charts & Figures | 253 |
| Segments covered | Test Type, Technology, End-use, and Region |
|---|
| Growth Drivers | - Increasing prevalence of cervical cancer
- Rising number of people suffering from Human Papillomavirus (HPV)
- Growing awareness regarding cervical cancer screening among women
- Surge in technological advancements regarding HPV and PAP test
|
|---|
| Pitfalls & Challenges | - Lack of reimbursement scenario
- Stringent regulatory framework
|
|---|
The strong presence of stringent regulatory frameworks may act as a major restraint for the industry growth. The lack of uniformity within the regulatory frameworks is giving rise to uncertainty for several firms that develop and market HPV and PAP tests given the variation of requirements and standards offered by different regulatory bodies, further delaying the product developments and market approvals. The increasing complexity of these regulations will also limit the deployment of HPV and PAP testing.
HPV Testing and PAP Test Market Analysis
With respect to test type, the HPV testing market size is projected be worth USD 4.8 billion by 2032. The growth can be attributed to the increasing awareness regarding cervical cancer and the growing number of sexually active people. The rapidly expanding homosexual population along with the increasing number of individuals affected with weakened immune systems has stirred the higher risk of contracting HPV, subsequently amplifying the awareness regarding HPV-related illnesses.
HPV testing and PAP test market size from the liquid-based cytology technology segment will witness over 16% CAGR from 2023-2032. Liquid-based cytology is widely employed in cervical cancer screening and includes the collection of cervical cells and their placement in liquid-based mediums for analysis. This technology is increasingly adopted in PAP tests as an alternative to traditional testing methods where cells can be directly transferred onto glass slides for examination.
North America HPV testing and PAP test market is expected to surpass USD 3.5 billion by 2032 due to the surging healthcare expenditure in the region. The increasing awareness related to cervical cancer has made way for a large number of supportive government initiatives. The thriving need for early disease diagnosis and the subsequently rising implementation of HPV testing as well as PAP test will influence the regional industry growth.
HPV Testing and PAP Test Market Share
Some of the prominent HPV testing and PAP test industry players include
- Hologic
- Quest Diagnostics
- Abbott Laboratories
- Becton Dickinson & Company
- Onco Health Corporation
- Arbor Vita Corporation
- Femasys
- Qiagen N.V.
- Seegene
- Mylab Discovery Solutions Pvt. Ltd.
HPV Testing and PAP Test Industry News
- In February 2023, the U.S. FDA granted approval to BD’s Onclarity HPV Assay deployed along with ThinPrep PAP test for detecting HPV types 16,18, 45 as well as genotypes 31, 51, 52, 56/59/66, 33/58, and 35/39/68.
- Roche Diagnostics, in June 2022, introduced a new HPV self-sampling solution in a bid to expands its cancer screening portfolio. The new solution assists patients in privately collecting the screening samples for HPV at healthcare facilities under the supervision of healthcare workers.
The HPV testing and PAP test market research report includes in-depth coverage of the industry with estimates & forecast in terms of revenue in USD Million and volume in Units from 2018 to 2032 for the following segments
Click here to Buy Section of this Report
By Test type
- Human papillomavirus (HPV) testing
- Papanicolaou (PAP) test
- Co-testing
By Technology
- Polymerase chain reaction (PCR)
- Liquid-based cytology
- Immunodiagnostics
- Hybrid capture
- Others
By End-use
- Hospitals
- Diagnostic centers
- Others
The above information is provided for the following regions and countries
- North America
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Russia
- Poland
- Switzerland
- The Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Thailand
- Indonesia
- Latin America
- Brazil
- Mexico
- Argentina
- Columbia
- Chile
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Israel




