Medical Device Testing Services Market Size - By Services (Biocompatibility Tests, Chemistry Test, Microbiology & Sterility Testing, Package Validation), By Phase (Preclinical, Clinical), Global Forecast, 2023 – 2032
Medical Device Testing Services Market Size - By Services (Biocompatibility Tests, Chemistry Test, Microbiology & Sterility Testing, Package Validation), By Phase (Preclinical, Clinical), Global Forecast, 2023 – 2032
Published Date: August - 2024 | Publisher: MIR | No of Pages: 240 | Industry: Healthcare | Format: Report available in PDF / Excel Format
View Details Buy Now 2890 Download Free Sample Ask for Discount Request CustomizationMedical Device Testing Services Market Size
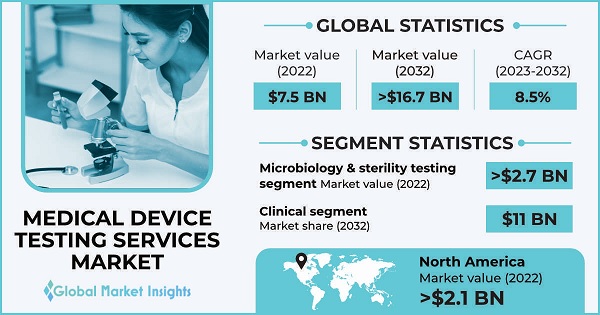
Medical Device Testing Services Market size was valued at around USD 7.5 billion in 2022 and it is anticipated to reach over 16.7 billion by 2032 at a CAGR of 8.5%. Rising complexity of medical devices, advancements in testing technologies, stringent regulatory requirements and focus on patient safety among others are key trends shaping the market growth.
As medical devices become more advanced and complex, their testing requirements also become more intricate. Manufacturers often lack the resources and expertise to perform these specialized tests in-house, leading them to rely on third-party testing services. Additionally, healthcare professionals, including doctors and surgeons, are becoming more conscious of the importance of using approved and tested medical devices. As a result, they are more likely to recommend and use devices that have undergone thorough testing and certification.
| Report Attribute | Details |
|---|---|
| Base Year | 2022 |
| Medical Device Testing Services Market Size in 2022 | 7.5 Billion USD |
| Forecast Period | 2023 to 2032 |
| Forecast Period 2023 to 2032 CAGR | 8.5% |
| 2032 Value Projection | 16.7 Billion USD |
| Historical Data for | 2018 to 2022 |
| No. of Pages | 130 |
| Tables, Charts & Figures | 190 |
| Segments covered | Services, Phase and Region |
| Growth Drivers |
|
| Pitfalls & Challenges |
|
Patient safety is of utmost importance in the healthcare industry. Thorough testing of medical devices helps identify potential risks and ensures that only safe and effective products reach the market, benefiting patients and healthcare providers. As the medical industry continues to evolve, the need for reliable and comprehensive testing will remain a critical factor for ensuring patient safety and market success for manufacturers.
Medical device testing services refer to the comprehensive range of tests and evaluations conducted on medical devices to ensure their safety, efficacy, and compliance with regulatory standards before they are approved for use in the market. These services are crucial in the medical industry as they help identify potential risks associated with the devices and confirm their performance and reliability.
However, while stringent regulations can drive demand for testing services, they can also create obstacles for some manufacturers. Meeting complex regulatory requirements can be time-consuming and costly, particularly for companies operating in multiple countries with varying regulatory frameworks. Comprehensive testing of medical devices can be expensive, especially when using advanced technologies and specialized equipment. This cost burden may deter some manufacturers, particularly smaller ones, from seeking professional testing services and could lead to delays in bringing products to market.
Further, some medical device manufacturers, particularly smaller or newer companies, may not fully understand the importance of comprehensive testing or the regulatory requirements. This lack of awareness can hinder the growth of the market.
COVID-19 Impact
The COVID-19 pandemic was like a thunderbolt for companies that test medical devices. It jolted their operations, smashing supply chains into smithereens. They struggled to get their hands on essential equipment and materials, like fragile glass vials and potent chemicals. The slowdown in testing created a backlog, with companies scrambling to meet the rising demand. The pandemic also pinched the pockets of medical device manufacturers. Forced to tighten their budgets, they trimmed expenses, including those on testing services. Adding insult to injury, governments were laser-focused on combating the pandemic, putting the approval process for new devices on the back burner. This setback was a double whammy for both testing companies and device makers eagerly awaiting the release of their products.
However, the pandemic increased the demand for specific medical devices such as ventilators, personal protective equipment (PPE), diagnostic tests, and remote patient monitoring devices. This surge in demand drove the need for testing services related to these critical products. It also prompted the adoption of remote and virtual testing methods where possible, allowing some testing service providers to continue operations despite physical restrictions. While the initial disruptions were challenging, the increased focus on healthcare preparedness and the accelerated innovation in the medical device industry have opened new avenues for growth in the testing services sector.
Medical Device Testing Services Market Trends
Stricter approval norms and regulations imposed by regulatory authorities serve several essential purposes, and their emphasis has a direct impact on the demand for testing services. The primary objective of strict approval norms is to ensure the safety of patients and users of medical devices. By mandating comprehensive testing and evaluation of devices, regulatory bodies aim to identify and mitigate potential risks associated with these products. The focus on patient safety increases the demand for testing services to thoroughly assess the devices before they reach the market.
Additionally, adherence to stringent approval norms helps reduce the likelihood of product recalls and legal liabilities for manufacturers. By ensuring that medical devices have undergone thorough testing, potential issues and risks are identified and addressed early in the development process, minimizing the chances of defects and failures after the devices are on the market.
Further, stricter approval norms push manufacturers to invest in research and innovation to meet regulatory requirements. This, in turn, stimulates the development of novel and more advanced medical devices. As manufacturers innovate, they seek specialized testing services to validate the safety and performance of these cutting-edge products. Thus, the growing focus on strict approval norms is instrumental in driving the market progress for medical device testing services.
Medical Device Testing Services Market Analysis

When it comes to testing medical devices, there are a few main types of serviceschecking how well they interact with living tissue (biocompatibility), making sure they're not contaminated with germs (microbiology and sterility), testing their chemical composition, and ensuring their packaging is up to snuff (package validation). Out of all these, testing for germs is a big deal. It's super important to make sure medical devices, especially ones that go into or near patients, don't have any nasty bugs on them. Keeping things sterile and germ-free helps prevent infections and other problems, which is key for keeping patients safe. Even the big shots like the FDA and EMA know how important this is. They require medical device manufacturers to test their products for germs before they can sell them.
Moreover, factors such as advancements in implantable medical devices, rise in point-of-care testing devices, infection prevention in healthcare settings and quality control are further propelling the segmental progression.

Medical device testing services are like checkpoints during the development journey of a new device. They help ensure it's safe and effective before it reaches patients. The testing process goes through different stages called phases. Clinical testing is the main event. Here, the device gets put to the test in real-life settings, with real people. Clinical testing is huge. In 2022, it brought in the most money, and it's only expected to grow bigger in the coming years, reaching a whopping 11 billion dollars by 2032. During clinical testing, data flows like a river. It needs to be collected carefully, managed properly, and analyzed in-depth. Testing services step up to the plate, making sure the data is accurate and trustworthy. These services play a critical role, ensuring the safety and effectiveness of medical devices that will ultimately help improve patients' lives.
Additionally, clinical testing may uncover areas for device improvement and optimization. Testing services help manufacturers iterate and refine the device design based on clinical trial outcomes.

The North America medical device testing services market was valued at over USD 2.1 billion in 2022. The significant regional share is attributed to the fact that North America is at the forefront of technological advancements in the medical device industry. The region is a hub for innovation and research, leading to the development of cutting-edge medical devices that require extensive testing services to support their regulatory approval and commercialization. Stringent regulatory environment in the U.S. overseen by the Food and Drug Administration (FDA), necessitate thorough testing and evaluation before medical devices can be approved for market entry.
Further, the region boasts a large and diverse medical device market, serving a wide range of healthcare needs. This diversity spans from diagnostic equipment and surgical instruments to implantable devices and remote patient monitoring solutions, all of which require testing services.
Medical Device Testing Services Market Share
Some of the leading players operating in the medical device testing services market include
- Charles River Laboratories
- American Preclinical Services
- North America Science Associates Inc
- Element Materials Technology
- WuXiAppTec Group
- Eurofins Scientific
- Labcorp (Toxikon, Inc),
- TÜV SÜD AG
These major market players are involved in strategic developments including mergers and acquisition, product launches, and collaborations, to expand and strengthen their business reach.
Medical Device Testing Services Industry News
- In March 2023, ARCHIMED, a leading investment firm focused on healthcare industries funded North America Science Associates Inc. (NAMSA) to acquire SUAZIO, a marketing consultancy firm specializing in the commercialization of new medical devices. This development will provide NAMSA with immediate cross selling opportunities in the industry, thereby enhancing its business reach.
- In May 2022, Eurofins BioPharma Product Testing has acquired Human Factors MD, LLC, a testing company for pharmaceutical and medical device firms. This development will allow the company to expand its service portfolio and better serve its customers at the same time improving its sales prospectus.
Medical device testing services market research report includes an in-depth coverage of the industry with estimates & forecast in terms of revenue (USD Million) from 2018 to 2032, for the following segments
Click here to Buy Section of this Report
By Services, 2018 - 2032 (USD Million)
- Biocompatibility Tests
- Chemistry Test
- Microbiology & Sterility Testing
- Package Validation
By Phase, 2018 - 2032 (USD Million)
- Preclinical
- Clinical
The above information is provided for the following regions and countries
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Spain
- Italy
- Poland
- Sweden
- The Netherlands
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Indonesia
- Philippines
- Vietnam
- Latin America
- Brazil
- Mexico
- Argentina
- Columbia
- Peru
- Chile
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Turkey
- Israel
- Iran
Related Reports
- Veterinary Endoscopes Market – By Product Type (Flexible [Video, Fibre-optic], Rigid), Procedure Type (Gastroduodenosc...
- Pet Wearable Market Size - By Product (Smart Collar, Smart Vest, Smart Harness, Smart Camera), By Technology (RFID Devic...
- Swine Artificial Insemination Market – By Product & Service (Semen {Fresh, Frozen}, Insemination Instruments {Catheter...
- Pet Tech Market - By Product (Pet Wearables, Smart Pet Crates & Beds, Smart Pet Doors, Smart Pet Feeders & Bowls, Smart ...
- Ovine and Caprine Artificial Insemination Market – By Type (Equipment, Semen, Reagents & Kits, Services) Animal Type (...
- Bovine Artificial Insemination Market – By Type (Services, Semen [Normal, Sexed], Equipment, Reagents & Kits), Techniq...
Table of Content
FAQ'S
For a single, multi and corporate client license, the report will be available in PDF format. Sample report would be given you in excel format. For more questions please contact:
Within 24 to 48 hrs.
You can contact Sales team (sales@marketinsightsresearch.com) and they will direct you on email
You can order a report by selecting payment methods, which is bank wire or online payment through any Debit/Credit card, Razor pay or PayPal.
Discounts are available.
Hard Copy